
Engineered Bacterial Vesicles to Combat Antimicrobial Resistance
Researchers identify novel surface-displaying proteins in extracellular vesicles derived from lactic acid bacteria
Pathogenic bacteria like Staphylococcus aureus pose serious threats to public health. Addressing the growing concern of antimicrobial resistance, researchers at Pusan National University, Korea, have developed a novel drug delivery platform using engineered extracellular vesicles (EVs) derived from Lacticaseibacillus paracasei. By displaying endolysins—enzymes that break down bacterial cell walls—on the EV surface, the team offers a promising new strategy to selectively eliminate harmful bacteria.
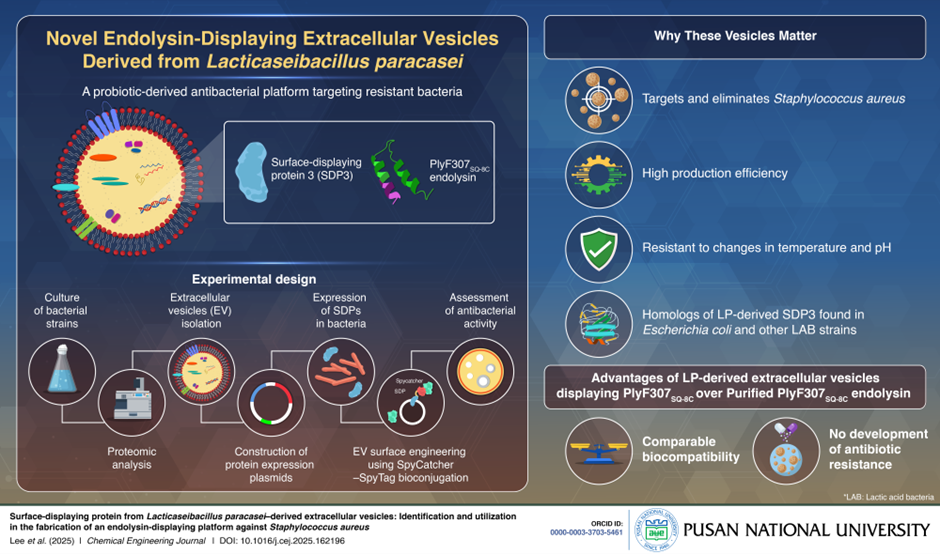
Image title: Extracellular vesicles displaying endolysins to specifically target and eliminate Staphylococcus aureus
Image caption: Pusan National University researchers developed novel extracellular vesicle (EV)-based platform to target harmful bacteria. The tailored EVs derived from Lacticaseibacillus paracasei contain a surface-displaying protein that carries PlyF307SQ-8C endolysin to target S. aureus.
Image credit: Professor Kwang-sun Kim from Pusan National University, Korea
License type: Original Content
Usage restrictions: Cannot be reused without permission
Bacteria are ubiquitous microscopic organisms capable of rapid growth. While beneficial strains like lactic acid bacteria (LAB) promote gut health and food preservation, pathogenic bacteria such as Escherichia coli and Staphylococcus aureus can cause severe infections. These harmful microbes produce toxins and enzymes that compromise health and, increasingly, show resistance to conventional antibiotics.
In recent years, scientists have explored alternative approaches to tackle pathogenic bacteria. Among them, endolysins—enzymes that degrade bacterial cell walls—have emerged as potent tools. These proteins, often derived from bacteriophages or engineered microbes, offer specificity in targeting pathogens. However, their widespread use is limited by challenges such as high production costs, instability during storage or circulation, and susceptibility to enzymatic degradation.
To address this research gap, researchers from Pusan National University, Korea have turned their attention to extracellular vesicles (EVs)-membrane-bound nanoparticles released by cells that transport biologically active molecules like proteins or nucleic acids. They engineered EVs derived from LAB to carry pathogen-specific endolysins on their surface. Their findings were made available online on 2 April, 2025 and published in volume 512 of Chemical Engineering Journal on 15 May, 2025. The research outlines the discovery and application of a novel surface-displaying protein found on EVs from Lacticaseibacillus paracasei.
In their study, the scientists initially cultured L. paracasei (LP)-a strain of LAB bacteria in the laboratory and then collected the EVs via high-speed centrifugation and isolation techniques. Subsequently, the fraction of proteins bound to EVs was subjected to comprehensive proteomic analysis. During further experiments to map the functions of proteins using advanced bioinformatics tools, the team identified 13 surface-displaying proteins (SDPs) associated with EVs derived from LP.
Explaining the significance of the present study, Prof. Kim says, “To date, no SDPs from the EVs of LAB species have been characterized. Now, for the first time, our group has identified a novel SDP named LP-SDP3 from the extracellular vesicles of L. paracasei. Additionally, we observed homologous proteins to SDP3 in E. coli and other LAB strains, with the SDP function conserved across these species”.
Inspired by their findings, the researchers went a step further and incorporated PlyF307SQ-8C, an endolysin that specifically targets S. aureus bacteria, into EVs with LP-SDP3. Remarkably, these EVs displaying PlyF307SQ-8C via the LP-SDP3 protein could selectively target and eliminate S. aureus. Furthermore, these engineered EVs were resistant to changes in temperature and pH, and did not induce antimicrobial resistance, while maintaining a similar safety profile compared to purified PlyF307SQ-8C endolysin.
“Engineered EVs derived from LAB can be produced on a large-scale and reduces the need for expensive protein purification technologies,” comments Prof. Kim. “In 5 to 10 years, this research could help reshape the way we treat infections, preserve food, and manufacture biological therapies—shifting away from antibiotics toward safe, smart, and sustainable bioengineered alternatives”.
Taken together, the identification of LP-SDP3 protein and its use in developing a novel, safe, and efficient EV-based platform can transform the landscape of antibacterial therapies.
Reference
Title of original paper: Surface-displaying protein from Lacticaseibacillus paracasei–derived extracellular vesicles: Identification and utilization in the fabrication of an endolysin-displaying platform against Staphylococcus aureus
Journal: Chemical Engineering Journal
DOI: 10.1016/j.cej.2025.162196
About Pusan National University
Pusan National University, located in Busan, South Korea, was founded in 1946 and is now the No. 1 national university of South Korea in research and educational competency. The multi-campus university also has other smaller campuses in Yangsan, Miryang, and Ami. The university prides itself on the principles of truth, freedom, and service and has approximately 30,000 students, 1,200 professors, and 750 faculty members. The university comprises 14 colleges (schools) and one independent division, with 103 departments in all.
Website: https://www.pusan.ac.kr/eng/Main.do
About the author
Dr. Kwang-sun Kim is a Professor of Chemistry at Pusan National University (PNU), Korea. His research focuses on understanding antibiotic resistance and developing innovative antimicrobial platforms, including nanomaterials and extracellular vesicle-based vaccines and therapeutics. He received his Ph.D. in Biochemistry from KAIST in 2004 and completed postdoctoral training at KAIST and in Stanley N. Cohen’s lab at Stanford University. From 2009 to 2015, he served as a Senior Researcher at the Korea Research Institute of Bioscience and Biotechnology (KRIBB). His work integrates biomedical and environmental applications of advanced vaccine and antimicrobial technologies.
Lab Website: https://rnabiochem.wixsite.com
ORCID id: 0000-0003-3703-5461

 PURCS_157_Infographic_final.jpg
(835KB)
PURCS_157_Infographic_final.jpg
(835KB)