
Embedded 3D-Coaxial Bioprinting of Stenotic Brain Vessels with a Mechanically Enhanced Extracellular Matrix Bioink for Investigating Hemodynamic Force-Induced Endothelial Responses
Hemodynamic Stress and Cerebrovascular Disease Modeling
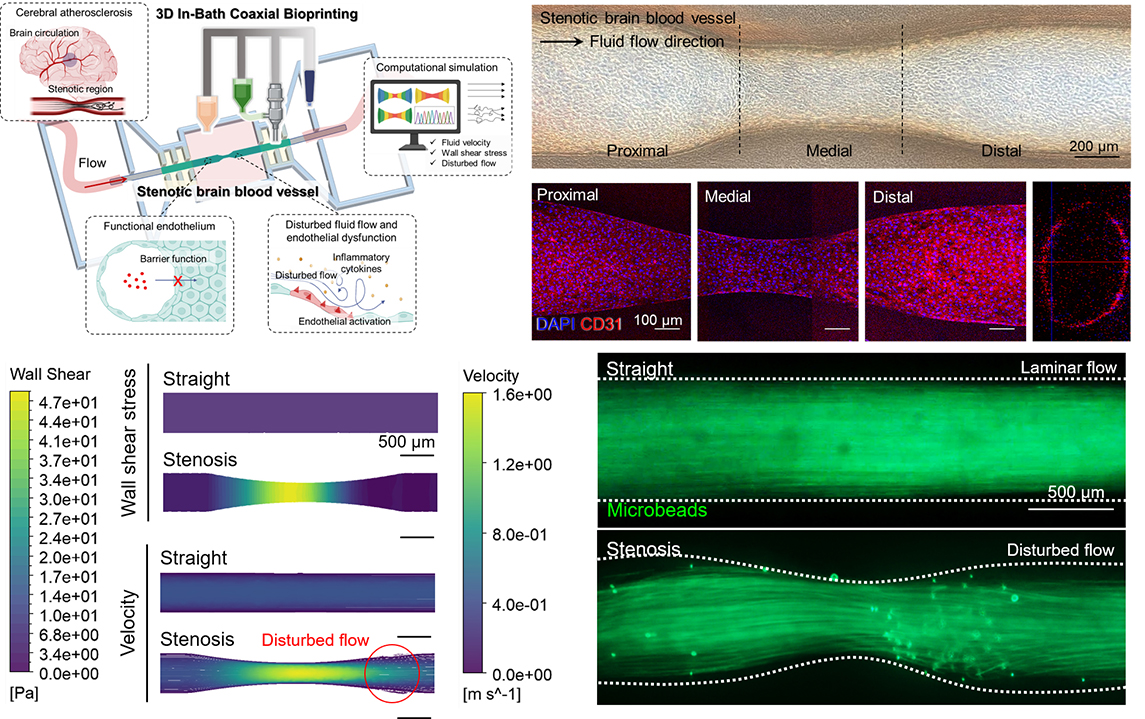
Hemodynamic forces such as shear stress and disturbed flow play a crucial role in cerebrovascular diseases including atherosclerosis and stroke. However, existing in vitro models have limited ability to replicate pathological flow environments and the resulting endothelial responses. To address this challenge, the research team developed a physiologically relevant model of stenotic brain vessels capable of mimicking disease-specific flow dynamics and inflammation.
Embedded 3D Coaxial Bioprinting and Hybrid Bioink Development
The team applied an embedded 3D coaxial bioprinting technique to fabricate stenotic vascular structures with tunable inner diameters (250–500 μm). To enhance the mechanical stability of the vessels, a hybrid bioink was developed by combining vascular tissue-derived decellularized ECM (VdECM) with collagen and alginate. Rheological and mechanical testing demonstrated that this formulation achieved a ~65-fold increase in dynamic modulus compared to VdECM alone, enabling stable, perfusable vessel fabrication.
Replication of Pathological Flow and Endothelial Inflammation
Using computational fluid dynamics (CFD) simulations, the model reproduced disturbed flow conditions characteristic of stenotic regions, including areas of low shear stress and flow recirculation. When human endothelial cells (HUVECs and HBMECs) were cultured within the printed vessels, they formed functional barriers expressing junctional proteins such as CD31, ZO-1, and VE-cadherin. Under disturbed flow, inflammatory markers ICAM-1 and VCAM-1 were upregulated by ~2.2-fold and ~1.5-fold, respectively, confirming shear-induced endothelial activation.
Quantitative Platform for Disease Modeling and Drug Testing
This study establishes a quantifiable and reproducible platform for investigating the interplay between vascular geometry, hemodynamics, and cellular inflammation. Unlike traditional microfluidic systems, the bioprinted model allows for precise structural customization and the use of ECM-derived bioinks with tissue-specific properties. The platform can be further extended for personalized disease modeling, therapeutic screening, and implantable graft development.
Future Directions
The modular and scalable nature of this bioprinting system enables its application to a wide range of vascular disease models beyond the brain, including coronary and peripheral arteries. Future studies may focus on integrating immune cells, neurovascular components, and in vivo implantation to evaluate long-term functionality and translational potential.
- Authors (Pusan National University): · Byoung Soo Kim (School of Biomedical Convergence Engineering)
· Min-Ju Choi (Department of Biomedical Convergence Engineering)
- Title of original paper: Embedded 3D-Coaxial Bioprinting of Stenotic Brain Vessels with a Mechanically Enhanced Extracellular Matrix Bioink for Investigating Hemodynamic Force-Induced Endothelial Responses
- Journal: Advanced Functional Materials
- Web link: https://doi.org/10.1002/adfm.202504276
- Contact e-mail: bskim7@pusan.ac.kr

 948김병수교수1-1.jpg
(723KB)
948김병수교수1-1.jpg
(723KB)