
Pusan National University Researchers Explain the Mechanism Behind High Anisotropic Evaporation Rate of Zinc Oxide Polar Surface
The instability of the zinc oxide polar surface is key to understanding this phenomenon, finds a new study
The high rate of anisotropic evaporation of the zinc oxide (ZnO) (0001) polar surface has been long known but remained unexplained. Now, a group of researchers from Korea, Germany, and France has explained this phenomenon using atomic-scale observations and computational simulations. The results show that a polarity-driven surface disorder is responsible for driving the fast evaporation and growth of ZnO along the [0001] direction. The finding could open doors to more efficient and durable catalysts.
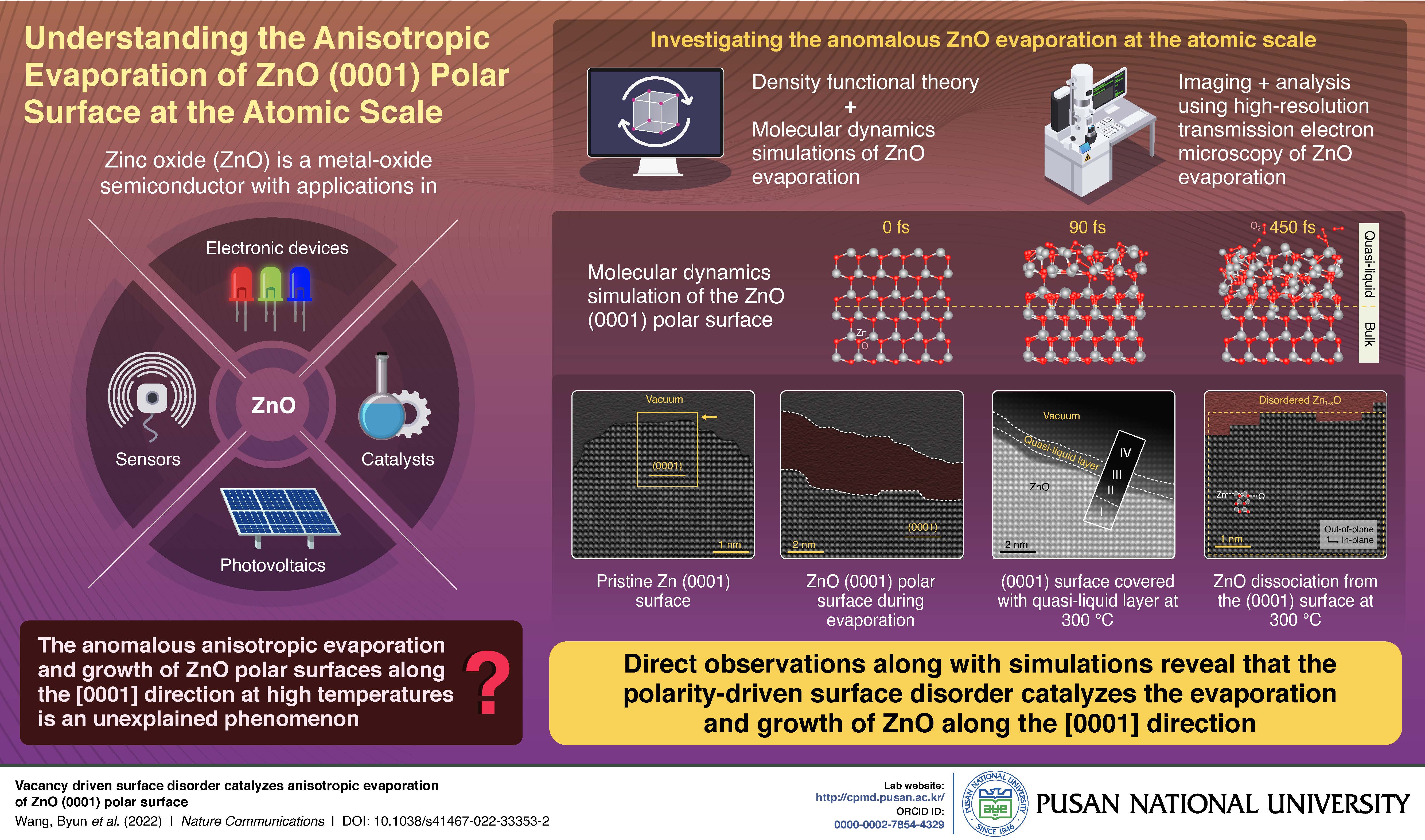
Zinc oxide (ZnO) is a popular metal-oxide semiconductor owing to its applicability in electronic devices, photovoltaics, sensors, and catalysis of chemical reactions. However, a curious phenomenon has made ZnO particularly interesting to scientists: the rate of evaporation of the ZnO (0001) polar surface at high temperatures is observed to be much higher than that predicted by thermodynamic calculations. So far, no satisfactory explanation of the observed high evaporation rate exists. But, scientists believe that it could be related to the intrinsic instability of the ZnO polar surface.
Fortunately, a team of scientists led by Associate Professor Jaekwang Lee from Pusan National University, Korea have now offered insights into this phenomenon by looking at what goes on during the evaporation at the atomic scale. Their research was made available online on September 24, 2022 and published in Volume 13 of the journal Nature Communications. “The high evaporation rate of ZnO polar surfaces, especially along the [0001] direction, has long remained a mystery. An understanding of the underlying mechanism, while important in its own right, could also help us design novel catalytic materials that are more efficient and durable,” says Dr. Lee, explaining the team’s motivation behind the study.
To observe the dynamic processes at the atomic level, the team used high-resolution transmission electron microscopy to image the (0001) polar surface. They observed that the evaporation rate of the (0001) surface went up significantly at 300 °C following a spontaneous formation of a catalytic quasi-liquid layer. Additionally, they found that ZnO dissociated from the surface at this temperature.
To make sense of their findings, the team then performed density functional theory calculations along with molecular dynamics simulations of the ZnO slab with the quasi-liquid layer. They found that the formation of the structurally disordered and Zn-deficit quasi-liquid layer was due to the inward diffusion of Zn vacancies on the (0001) polar surface. Further, the dissociation of ZnO at 300 °C was controlled by this quasi-liquid layer, which established steady-state reactions with Zn and oxygen vapors in the ZnO crystal and the atmosphere. The quasi-liquid layer catalyzed the dissociation of the ZnO lattice by injecting Zn vacancies and facilitating the desorption of oxygen molecules.
“Our theoretical predictions combined with atomic-scale observations reveal that the polarity-driven surface disorder is the key structural feature responsible for the fast evaporation and growth along the c-axis of ZnO, in particular the [0001] direction,” highlights Dr. Lee. “This insight provides a better understanding of the fundamental principles governing surface phenomena, and a basis for the design and development of precise thin-film deposition techniques that could have potential implications for electronics, photovoltaics, and sensor technologies,” he concludes.
It certainly is a win-win for both science and technology!
Reference
Authors: Zhen Wang1, Jinho Byun2, Subin Lee1,3, Jinsol Seo1,4, Bumsu Park1,5, Jong Chan Kim6, Hu Young Jeong6, Junhyeok Bang7,*, Jaekwang Lee2,* & Sang Ho Oh1,4,*
Title of original paper: Vacancy driven surface disorder catalyzes anisotropic evaporation of ZnO (0001) polar surface
Journal: Nature Communications
DOI: 10.1038/s41467-022-33353-2
Affiliations: 1Department of Energy Science, Sungkyunkwan University, Korea
2Department of Physics, Pusan National University, Korea
3Institute of Applied Mechanics–Materials and Biomechanics, Karlsruhe Institute of Technology
4Department of Energy Engineering, KENTECH Institute for Energy Materials and Devices, Korea Institute of Energy Technology (KENTECH)
5CEMES-CNRS
6UNIST Central Research Facilities, Ulsan National Institute of Science and
Technology (UNIST)
7Department of Physics, Chungbuk National University
*Corresponding authors’ emails: jbang@cbnu.ac.kr, jaekwangl@pusan.ac.kr, shoh@kentech.ac.kr
Your Press Release Source
Pusan National University
About Pusan National University
Pusan National University, located in Busan, South Korea, was founded in 1946, and is now the no. 1 national university of South Korea in research and educational competency. The multi-campus university also has other smaller campuses in Yangsan, Miryang, and Ami. The university prides itself on the principles of truth, freedom, and service, and has approximately 30,000 students, 1200 professors, and 750 faculty members. The university is composed of 14 colleges (schools) and one independent division, with 103 departments in all.
Website: https://www.pusan.ac.kr/eng/Main.do
About the author
Prof. Jaekwang Lee is an Associate Professor in the Department of Physics at Pusan National University (PNU), Korea. He received his Ph.D. in Physics at the University of Texas at Austin, USA, in 2010. He was awarded The Young Scientist Award from the Physics and Chemistry of Surface and Interfaces (PCSI) in 2011. From 2010 to 2014, he worked as a postdoctoral researcher at the Oak Ridge National Laboratory, USA. In 2015, he joined the Department of Physics at PNU. His research interests include first-principles study of the interfaces, spectroscopy simulations, and materials design for energy applications.
Lab website: http://cpmd.pusan.ac.kr
ORCID id: 0000-0002-7854-4329

 PURCS_86_Infographic_final.jpg
(1MB)
PURCS_86_Infographic_final.jpg
(1MB)